Hagemann lab
Metabolic regulation of carbon allocation from the Calvin-Benson-Bassham cycle under different CO2 supply
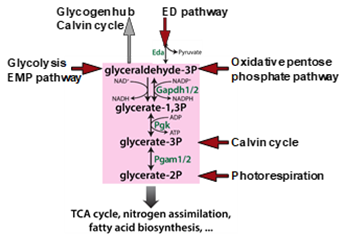
Cyanobacterial CO2 fixation must acclimate to varying conditions. Under different CO2 availability, defined metabolic changes were detected without corresponding alterations in gene expression that points at a dedicated role of biochemical regulation1. The Calvin cycle as main CO2-fixing route plays the central role in the cyanobacterial carbon metabolism. Organic carbon can be taken out from the Calvin cycle into two directions: by phosphoglycerate mutases (PGAMs) towards lower glycolysis and TCA cycle or glyceraldehyde-3-phosphate (Gap) can be converted into glycogen as main carbon storage under carbon excess conditions (Fig. 1). In the dark or under specific conditions such as shift from high to low Ci in the light, glycogen can be mobilized via three different routes, oxidative pentosephosphate pathway, the glycolytic Entner-Doudoroff (ED) or Embden-Meyerhof-Parnas (EMP) pathways, which all merge in the so-called triosephosphate hub2. The different routes in carbon anabolism and catabolism are performed in the non-compartmented cyanobacterial cell, hence, proper regulation is needed to avoid futile cycles. For the carbon flow in the direction of the lower glycolysis, PGAM1 is mainly responsible, the activity of which is switched on or off by the regulatory protein PirC3, while the other PGAM isoenzymes tend not to be involved. Further experiments showed that Gap-dehydrogenase isoenzyme 1, GapDH1 cannot use NADPH2 and participates in heterotrophic metabolism. The GapDH2 is involved in the Calvin cycle and is mainly regulated by the protein CP12. Investigations of the phosphoproteome showed that CP12 and subunits of a bicarbonate transporter are dephosphorylated under low CO2 conditions, while the phosphorylation of the protein kinase SpkC increases4.
1. Jablonsky J, Papacek S, Hagemann M (2016) Different strategies of metabolic regulation in cyanobacteria: from transcriptional to biochemical control. Sci Rep 6:33014
2. Chen X, Schreiber K, Appel J, Makowka A, Fähnrich B, Roettger M, Hajirezaei MR, Sönnichsen FD, Schönheit P, Martin WF, Gutekunst K (2016) The Entner-Doudoroff pathway is an overlooked glycolytic route in cyanobacteria and plants. Proc Natl Acad Sci USA 113:5441-5446
3. Orthwein T, Scholl J, Spät P, Lucius S, Koch M, Macek B, Hagemann M, Forchhammer K (2021) The novel PII-interactor PirC identifies phosphoglycerate mutase as key control point of carbon storage metabolism in cyanobacteria. Proc Natl Acad Sci USA 118:e2019988118
4. Spät P, Barske T, Maček B, Hagemann M (2021) Alterations in the CO2 availability induce alterations in the phospho-proteome of the cyanobacterium Synechocystis PCC 6803. New Phytol, https://nph.onlinelibrary.wiley.com/doi/10.1111/nph.17423
Principal Investigator

University of Rostock
Department of Plant Physiology
Einsteinstr. 3, D-18059 Rostock, Germany
martin.hagemannuni-rostock.de
Website
Members

nils.schmidtuni-rostock.de
Alumni

Alumni